Fireworks. The very word conjures images of dazzling light, booming sound, and celebratory spectacle. But beyond the ephemeral beauty lies a surprisingly consistent scientific foundation, one rooted in centuries of alchemical experimentation, meticulous observation, and a keen understanding of materials science – even before the formalization of those disciplines. This article will delve into the history of firework composition, tracing its evolution from ancient Chinese origins to the sophisticated displays of the Renaissance and beyond, highlighting the often-overlooked scientific principles at play.
From Ancient Origins to Alchemical Exploration
The story of fireworks begins in China, around the 9th century AD, with the accidental discovery of gunpowder. Legend attributes the discovery to alchemists searching for an elixir of immortality, a common pursuit in many ancient cultures. While they didn’t achieve their desired immortality, they stumbled upon a potent mixture of sulfur, charcoal, and saltpeter (potassium nitrate). Initially, this wasn’t used for celebratory displays; it was primarily employed in military applications – bombs, rockets, and early forms of firearms.
The key to gunpowder’s explosive power lies in its composition. Saltpeter acts as the oxidizer, providing the oxygen necessary for rapid combustion. Charcoal provides the fuel (carbon), and sulfur lowers the ignition temperature, making the mixture more easily flammable. The proportions were crucial, and early Chinese pyrotechnicians quickly learned through trial and error that specific ratios yielded different effects. This early experimentation, though driven by different goals, laid the groundwork for the precise formulations we see today.
The transmission of gunpowder technology to the West occurred gradually, likely along the Silk Road. By the 13th century, knowledge of gunpowder had reached Europe and the Middle East, sparking further experimentation. However, it wasn’t simply a matter of replicating the Chinese formula. European alchemists, influenced by their own philosophical traditions, began to modify and refine the composition, leading to a divergence in firework styles and techniques.
The Renaissance and the Rise of Pyrotechnic Art
The Renaissance witnessed a flowering of artistic and scientific inquiry, and fireworks were no exception. Italian and French pyrotechnicians, in particular, elevated firework displays to an art form. Figures like Bartolomeo Bianco and Vannoccio Buggieri, employed by the Medici family in Florence, were not merely makers of explosions; they were designers of spectacular theatrical events. These displays were often integrated into courtly celebrations, weddings, and political triumphs.
This era saw a significant expansion in the variety of firework effects. Simple star-shaped bursts evolved into intricate designs, including animals, architectural structures, and even mythological scenes. This required a deeper understanding of how different chemical compounds affected the color, duration, and trajectory of the fireworks.
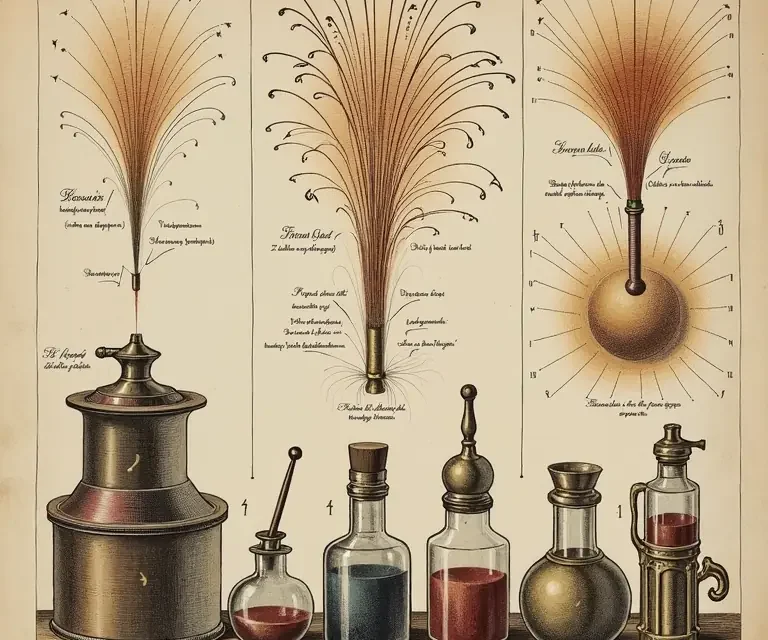
Color, in particular, presented a significant challenge. Early fireworks were largely limited to shades of white, orange, and yellow, derived from burning charcoal, sulfur, and iron filings. Achieving vibrant blues, greens, and violets required the introduction of new metallic salts. Strontium salts produced reds, barium salts yielded greens, copper salts created blues, and sodium salts gave yellow hues. The process wasn’t straightforward; the purity of the salts, their particle size, and their interaction with other components all influenced the final color. It’s fascinating to note how this aligns with principles of chemistry that wouldn’t be formally articulated for centuries.
The Science of Composition: Beyond Alchemy
While alchemy provided the initial impetus and methodology, the development of firework composition gradually moved towards a more empirical and scientific approach. Pyrotechnicians meticulously documented their experiments, recording the effects of different mixtures and refining their formulas based on observation. The understanding of deflagration (rapid combustion that travels through a material) versus detonation (an explosive shockwave) became crucial. Fireworks rely on deflagration, which allows for a controlled burn and the creation of visual effects, whereas detonation would simply result in a shattering explosion.
The composition of a typical aerial shell, the foundation of modern fireworks, illustrates this scientific complexity. An aerial shell typically consists of several key components:
- Black Powder (Gunpowder): Used as a lifting charge to propel the shell into the air and as a bursting charge to scatter the stars.
- Stars: Small pellets containing the colorant, fuel, and binder. The composition of the stars determines their color, brightness, and duration.
- Bursting Charge: A quantity of black powder that ignites upon reaching the desired altitude, breaking open the shell and scattering the stars.
- Time Delay Fuse: A fuse that delays ignition of the bursting charge, allowing the shell to reach sufficient altitude.
- Shell Casing: Typically made of cardboard or paper, providing structural integrity during flight.
The precise layering and arrangement of these components are critical for a successful display. The lifting charge must be powerful enough to overcome gravity and air resistance, while the bursting charge must be precisely calibrated to create the desired effect. The time delay fuse must function reliably, and the shell casing must withstand the stresses of flight and explosion.
The physics of projectile motion also plays a vital role. The angle of launch, the initial velocity, and air resistance all affect the trajectory of the shell. Experienced pyrotechnicians account for these factors to ensure that the fireworks burst at the optimal altitude and in the desired location. This isn’t dissimilar to the principles governing the flight of a cannonball or, indeed, the trajectory of a yo-yo – a surprisingly consistent application of physics across centuries! Read more about the physics of seemingly simple devices.
The Role of Binders and Stabilizers
Beyond the core components, the inclusion of binders and stabilizers is essential for creating a cohesive and reliable firework composition. Binders, such as dextrin (a starch-based substance) or gum arabic, hold the ingredients together, forming a solid mass that can be shaped into stars or other forms. Stabilizers, such as charcoal or graphite, help to prevent unwanted reactions and ensure that the firework burns smoothly and predictably.
The particle size of the ingredients also significantly impacts performance. Finely ground powders burn more quickly and completely than coarser particles, resulting in brighter and more intense colors. However, excessively fine powders can be more prone to static electricity buildup, increasing the risk of accidental ignition.
The Aesthetic Dimension: Choreography of Light and Sound
While the science of firework composition focuses on the chemical and physical properties of the materials, the art of pyrotechnics also encompasses an aesthetic dimension. Pyrotechnicians are not simply creating explosions; they are composing a visual and auditory spectacle. This requires a deep understanding of color theory, spatial arrangement, and timing.
The arrangement of stars within a shell, for example, determines the shape of the burst. By carefully positioning the stars, pyrotechnicians can create a wide range of effects, from simple circular bursts to complex geometric patterns. The timing of the bursts is also crucial. A well-choreographed display will build in intensity, culminating in a grand finale that leaves the audience breathless. This careful orchestration is reminiscent of the planning involved in carnival games, where understanding psychology is as important as the mechanics of the game. Explore the psychology behind games of chance and skill.
Modern Advancements and Continued Consistency
Modern firework technology has built upon the foundations laid by centuries of experimentation. Computer-controlled firing systems allow for precise timing and synchronization, enabling incredibly complex displays. New chemical compounds have been developed to expand the range of available colors and effects. However, the fundamental principles remain the same. The need for precise composition, controlled combustion, and careful choreography remains paramount.
The pursuit of novelty also drives innovation. Effects like comets (long-trailing stars), willows (graceful, weeping bursts), and peonies (spherical bursts with a fading trail) are constantly being refined and reimagined. The use of specialized chemicals, such as titanium for sparkling effects and flash powder for bright white bursts, adds to the visual richness of the displays.
Interestingly, the dedication to precision and intricate mechanisms in firework construction echoes the craftsmanship found in antique clockwork music boxes. Delve into the intricate world of clockwork mechanisms. Both fields require a deep understanding of materials, mechanics, and a commitment to meticulous detail.
The Enduring Legacy and Unexpected Connections
The story of firework composition is a testament to the power of human curiosity and ingenuity. What began as a search for immortality in ancient China evolved into a sophisticated art form that continues to captivate audiences worldwide. The journey from alchemy to modern pyrotechnics demonstrates how scientific principles can emerge from seemingly esoteric pursuits.
The meticulous markings and techniques employed by early pyrotechnicians also share a surprising consistency with the logic behind antique tool markings. Discover the hidden stories behind antique tool markings. Both fields reveal a dedication to craftsmanship, precision, and the preservation of knowledge through subtle but meaningful details.
Furthermore, the geometric precision required in designing firework bursts and patterns finds a parallel in the mathematical foundations of traditional origami. Explore the mathematical elegance of origami. Both art forms demonstrate how seemingly simple principles can yield complex and beautiful results.
The science of fireworks isn’t just about explosions; it’s about controlling energy, manipulating matter, and creating a fleeting moment of wonder. The consistency of the underlying principles, from the earliest Chinese experiments to the latest innovations, speaks to the enduring power of scientific inquiry and the human desire to illuminate the darkness.
 The Curious Mechanics of Automaton Birds: A History of Feathered Clockwork
The Curious Mechanics of Automaton Birds: A History of Feathered Clockwork  The Curious Mechanics of Celestial Globes: Mapping the Heavens in Miniature
The Curious Mechanics of Celestial Globes: Mapping the Heavens in Miniature  The Surprisingly Consistent Science of Historical Weather Vanes – Art, Meteorology & Directional Lore
The Surprisingly Consistent Science of Historical Weather Vanes – Art, Meteorology & Directional Lore  The Surprisingly Consistent Logic of Traditional Herbal Remedies: Beyond Folklore, a History of Observation
The Surprisingly Consistent Logic of Traditional Herbal Remedies: Beyond Folklore, a History of Observation  The Surprisingly Consistent Science of Early Map Projections: Distorting the World to Understand It
The Surprisingly Consistent Science of Early Map Projections: Distorting the World to Understand It  The Surprisingly Consistent Science of Candle Flame Dynamics: A History of Light, Wax, and Atmospheric Chemistry
The Surprisingly Consistent Science of Candle Flame Dynamics: A History of Light, Wax, and Atmospheric Chemistry